



INJECTION MANUFACTURER IN SIKKIM
Product Details:
- Indication Prescribed by medical professionals for targeted therapy
- Shelf Life 24-36 months from date of manufacture
- Packaging Type Vial/Ampoule
- Medicine Type Injection
- Physical Form Liquid
- Ingredients Pharmaceutical Grade Raw Material
- Treatments & Functions Used for various therapeutic treatments as per formulation
- Click to View more
INJECTION MANUFACTURER IN SIKKIM Price And Quantity
- 1000 Carton
- Available for domestic and international supply
- Glass or medical-grade plastic
- Sterile production environment, validated by external audits
- Available as per client formulation and needs
- For therapeutic use only under medical supervision
- Complies with Indian and international pharmacopeia guidelines
- Parenteral Administration
- Sikkim, India
- Single or multi-dose preparations available
- ISO, GMP Certified Manufacturing
INJECTION MANUFACTURER IN SIKKIM Product Specifications
- Varies as per product requirement
- Vial/Ampoule
- Store in a cool, dry place away from direct sunlight
- Liquid
- Pharmaceutical Grade Raw Material
- Used for various therapeutic treatments as per formulation
- Customized as per client specification
- Hospitals, Clinics, and Medical Institutions
- Prescribed by medical professionals for targeted therapy
- 24-36 months from date of manufacture
- Injection
- Available for domestic and international supply
- Glass or medical-grade plastic
- Sterile production environment, validated by external audits
- Available as per client formulation and needs
- For therapeutic use only under medical supervision
- Complies with Indian and international pharmacopeia guidelines
- Parenteral Administration
- Sikkim, India
- Single or multi-dose preparations available
- ISO, GMP Certified Manufacturing
Product Description
INJECTIONS
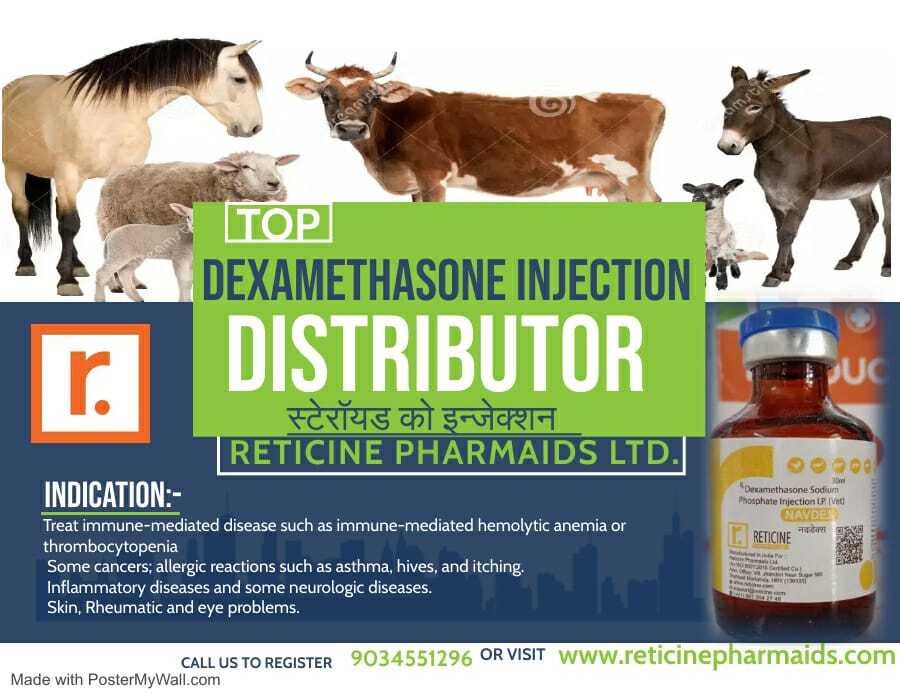
MELOXICAM, MELOXICAM PARACETAMOL, NIMESULIDE, NIMESULIDE PARACETAMOL, OXYTETRACYCLINE, PIROXICAM PARACETAMOL, IVERMECTION, GENTAMICIN, FLUNIXIN MEGLUMINE, ENROFLOXACIN, CLOPROSTENTOL, CHLOROHENIRAMINE MALEATE, BUTAPHOSPHAN CYNOCOBALAMIN, ATROPINE SULPHATE, AMIKACIN SULPHATE, DEXAMETHASONE, HYDROXY PROGESTERONE, ISOFLUPREDONE, KETOPROFEN,LEVOFLOXACIN, DICYCLOMINE, MECOBALAMIN, NATURAL PROGESTERONE, PHENIRAMINE, SODIUM ACID PHOSPHATE, SULPHADIMIDINE,VITAMINA, VITAMIN B COMPLEX, VITAMIN B COMPLEX WITH LIVER EXTRACT(B1,B2,B3,B12), VITAMINB1,B6,B12 ETC.
DRY POWDER INJECTION
CEFTRIAXONE, CEFTRIAXONE SULBACTUM, CEFOPARAZONE SULBACTUM, CEFOPERAZONE TAZOBACTUM, CEFTIOFUR, AMOXYCILLIN AND POTASSIUM CLAVULANATE, AMOXYCILLIN AND CLOXACILLIN, STREPTOPENICILLIN FOR SUSPENSION, AMOXYCILLIN AND CLOXACILLIN, CEFTRIAXONE TAZOBACTUM ETC.
Advanced Manufacturing in Sikkim
Leveraging state-of-the-art technology, our facility adheres strictly to ISO and GMP standards to manufacture sterile parenteral preparations. Rigorous validation by independent external audits underpins our commitment to sterility and quality in every batch produced.
Global Reach and Customization
We support both domestic and international clients, offering tailored solutionswhether in dose, formulation, or packaging. Our expertise ensures compliance with Indian and global pharmacopeia standards, making us an ideal partner for diverse therapeutic requirements.
Reliable, Safe Therapeutic Injections
All products undergo stringent quality control and are intended strictly for administration by medical professionals. They are safely packed for extended shelf life (24-36 months) and shipped with clear storage and usage instructions for hospitals, clinics, and other healthcare institutions.
FAQs of INJECTION MANUFACTURER IN SIKKIM:
Q: How does the Sikkim-based facility ensure sterility and quality of its injection products?
A: We maintain a sterile production environment validated by ISO and GMP certifications, complemented by periodic external audits. Each batch is thoroughly tested for quality, sterility, and compliance with Indian and international pharmacopeia.Q: What types of injections can be manufactured, and can they be customized?
A: We manufacture both single and multi-dose injections in liquid form, made from pharmaceutical-grade raw materials. Customization is available for dose, formulation, container material, and packaging to meet client and therapeutic needs.Q: What are the recommended storage instructions for your injection products?
A: Products should be stored in a cool, dry place away from direct sunlight to maintain efficacy and prolong shelf life, which ranges from 24 to 36 months after the date of manufacture.Q: Where can your injection products be supplied or exported?
A: Our injections are available for both domestic and international markets, delivered to hospitals, clinics, medical institutions, and other healthcare facilities as per client requirements.Q: What is the process for ensuring regulatory compliance during manufacturing?
A: We strictly adhere to Indian and international pharmacopeia guidelines, ensuring each product meets meticulous standards for safety, potency, and sterility, verified through documentation and audits.Q: When and how should these injections be used?
A: These injections are intended for therapeutic use only under medical supervision, following a prescription from a healthcare professional who determines the appropriate indication and dosage based on patient needs.Q: What benefits do your injection manufacturing services offer to clients?
A: Clients benefit from customized formulations, consistent quality controls, secure packaging, and regulatory compliance. Our services enhance the treatment capabilities of healthcare providers while ensuring product safety and efficacy.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+












 Send Inquiry
Send Inquiry Send SMS
Send SMS