



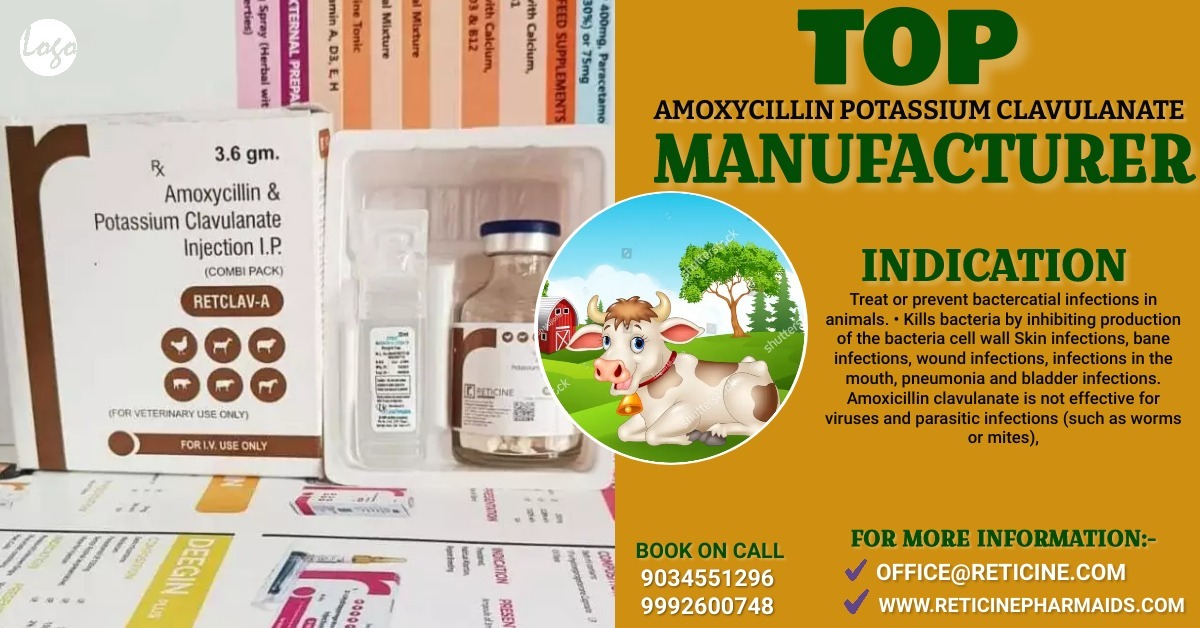

VETERINARY INJECTION MANUFACTURER IN MAHARASHTRA
Product Details:
- Packaging Type Glass Vial, Plastic Vial, or Ampoule
- Indication As indicated by the veterinary healthcare provider
- Shelf Life 24 - 36 months
- Medicine Type Veterinary Injection
- Physical Form Liquid
- Ingredients Pharmaceutical Grade Ingredients
- Treatments & Functions Treatment and prevention of specific animal diseases
- Click to View more
X
VETERINARY INJECTION MANUFACTURER IN MAHARASHTRA Price And Quantity
- 1000 Carton
- Maharashtra, India
- Registered under regulatory norms for veterinary pharmaceuticals
- Within safe range for animal administration
- Colourless or as specified for product type
- Batch number, manufacturing date, expiry date, and composition on the packaging
- To be administered by or under supervision of a qualified veterinarian
- For intramuscular or subcutaneous injection
- Sterile and ready-to-use
VETERINARY INJECTION MANUFACTURER IN MAHARASHTRA Product Specifications
- As per prescription or product label
- Animals (Cattle, Poultry, Pets, etc.)
- Glass Vial, Plastic Vial, or Ampoule
- 24 - 36 months
- Available in multiple volumes (e.g., 10ml, 30ml, 100ml)
- As indicated by the veterinary healthcare provider
- Store in a cool and dry place, away from direct sunlight
- Liquid
- Veterinary Injection
- Treatment and prevention of specific animal diseases
- Pharmaceutical Grade Ingredients
- Maharashtra, India
- Registered under regulatory norms for veterinary pharmaceuticals
- Within safe range for animal administration
- Colourless or as specified for product type
- Batch number, manufacturing date, expiry date, and composition on the packaging
- To be administered by or under supervision of a qualified veterinarian
- For intramuscular or subcutaneous injection
- Sterile and ready-to-use
Product Description
VETERINARY INJECTION
MELOXICAM, MELOXICAM PARACETAMOL, NIMESULIDE, NIMESULIDE PARACETAMOL, OXYTETRACYCLINE, PIROXICAM PARACETAMOL, IVERMECTION, GENTAMICIN, FLUNIXIN MEGLUMINE, ENROFLOXACIN ETC.
DRY POWDER INJECTION
CEFTRIAXONE, CEFTRIAXONE SULBACTUM, CEFOPARAZONE SULBACTUM, CEFOPERAZONE TAZOBACTUM, CEFTIOFUR, AMOXYCILLIN AND POTASSIUM CLAVULANATE, AMOXYCILLIN AND CLOXACILLIN ETC.
Cutting-Edge Veterinary Injection Manufacturing
Our facility in Maharashtra is equipped with advanced technology to ensure every injection meets exacting standards of sterility, efficacy, and safety for animal health. We conduct rigorous quality checks throughout the production process, ensuring each unit is suitable for medicinal use in diverse animal species, from cattle to companion pets.
Comprehensive Product Registration and Accreditation
Every veterinary injection is registered according to strict regulatory guidelines governing veterinary pharmaceuticals in India. This guarantees consistent quality, reliable performance, and regulatory approval for domestic and export markets. Our labeling system provides complete transparency about product composition, batch details, and shelf life.
FAQs of VETERINARY INJECTION MANUFACTURER IN MAHARASHTRA:
Q: How are these veterinary injections administered to animals?
A: These injections are designed for intramuscular or subcutaneous administration and should always be given by or under the supervision of a qualified veterinarian, following the prescribed dosage and guidelines on the product label.Q: What animals can benefit from these injections?
A: Our veterinary injections are suitable for various animals, including cattle, poultry, pets, and other livestock, depending on the specific disease or condition as indicated by a veterinary healthcare provider.Q: What safety precautions should be taken during administration?
A: It is important to ensure that the injection is administered only by a trained veterinarian. Always check the label for dosage, batch number, and expiry date, and follow recommended aseptic techniques to minimize risks.Q: Where should these injections be stored for maximum efficacy?
A: Store the injections in a cool, dry place away from direct sunlight, as recommended on the packaging, to maintain their stability and effectiveness throughout their 2436 month shelf life.Q: What information is provided on the product label?
A: The packaging includes comprehensive information such as the batch number, manufacturing date, expiry date, complete composition, and other relevant details to ensure traceability and informed usage.Q: When should these injections be used for animals?
A: Veterinary injections should be used as indicated by a qualified healthcare provider, typically for treatment or prevention of diagnosed diseases or as part of a health management program as prescribed.Q: What are the benefits of sourcing injections from a registered manufacturer in Maharashtra?
A: Purchasing from a registered manufacturer ensures compliance with regulatory standards, consistent product quality, and access to support for dealers, exporters, suppliers, and animal health professionals across India and abroad.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email













 Send Inquiry
Send Inquiry Send SMS
Send SMS