
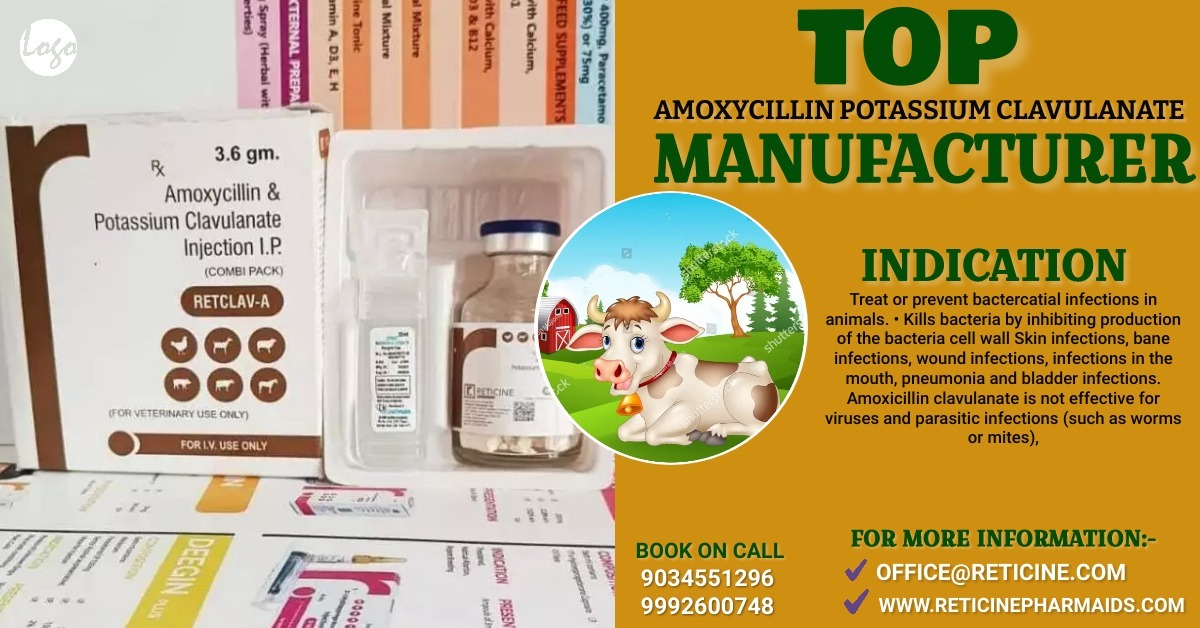




INJECTION MANUFACTURER IN BIHAR
Product Details:
- Packaging Type Sterile Vial/Ampoule
- Indication Specific to formulation (e.g., pain management, infection control, etc.)
- Shelf Life 24 months
- Medicine Type Injection
- Physical Form Liquid
- Ingredients Pharmaceutical Grade Ingredients
- Treatments & Functions Used for treatment of various medical conditions as advised by doctor
- Click to View more
INJECTION MANUFACTURER IN BIHAR Price And Quantity
- 1000 Carton
- Bulk & contract manufacturing available
- Available as per client requirement
- To be administered by qualified medical personnel only
- Clear or slightly colored liquid
- Manufactured in Bihar, India
- Intravenous (IV) or Intramuscular (IM) as prescribed
- Meets Indian Pharmacopeia Standards
- Sterile Injectable Solution
- Hospital, Clinical, and Institutional Use
- Glass or high-grade medical plastic
INJECTION MANUFACTURER IN BIHAR Product Specifications
- 24 months
- Specific to formulation (e.g., pain management, infection control, etc.)
- Adults, Children as directed by physician
- Liquid
- Injection
- Sterile Vial/Ampoule
- Customizable (as per prescription)
- Vial/ampoule (as per requirement)
- Store in a cool and dry place
- Pharmaceutical Grade Ingredients
- Used for treatment of various medical conditions as advised by doctor
- Bulk & contract manufacturing available
- Available as per client requirement
- To be administered by qualified medical personnel only
- Clear or slightly colored liquid
- Manufactured in Bihar, India
- Intravenous (IV) or Intramuscular (IM) as prescribed
- Meets Indian Pharmacopeia Standards
- Sterile Injectable Solution
- Hospital, Clinical, and Institutional Use
- Glass or high-grade medical plastic
Product Description
WE PROVIDE VETERINARY PCD FRANCHISE , VETERINARY THIRD PARTY MANUFACTURING , VETERINARY INJECTION MANUFACTURER, VETERINARY BOLUS MANUFACTURER, VETERINARY ANIMAL FEED SUPPLEMENT, OXYTETRACYCLINE INJECTION, TOP TEN VETERINARY COMPANY, AND WE PROVIDED VETERINARY INJECTION MANUFACTURERS IN DIFFERENT-2 STATES. LIKE, MAHARASHTRA,PUNJAB,HARYANA,WEST BENGAL,GUJARAT,KERALA,TELANGANA ETC. ALSO WE PROVIDED PET PRODUCTS.
INJECTIONS
MELOXICAM, MELOXICAM PARACETAMOL, NIMESULIDE, NIMESULIDE PARACETAMOL, OXYTETRACYCLINE, PIROXICAM PARACETAMOL, IVERMECTION, GENTAMICIN, FLUNIXIN MEGLUMINE, ENROFLOXACIN, CLOPROSTENTOL, CHLOROHENIRAMINE MALEATE, BUTAPHOSPHAN CYNOCOBALAMIN, ATROPINE SULPHATE, AMIKACIN SULPHATE, DEXAMETHASONE, HYDROXY PROGESTERONE, ISOFLUPREDONE, KETOPROFEN,LEVOFLOXACIN, DICYCLOMINE, MECOBALAMIN, NATURAL PROGESTERONE, PHENIRAMINE, SODIUM ACID PHOSPHATE, SULPHADIMIDINE,VITAMINA, VITAMIN B COMPLEX, VITAMIN B COMPLEX WITH LIVER EXTRACT(B1,B2,B3,B12), VITAMINB1,B6,B12 ETC.
DRY POWDER INJECTION
CEFTRIAXONE, CEFTRIAXONE SULBACTUM, CEFOPARAZONE SULBACTUM, CEFOPERAZONE TAZOBACTUM, CEFTIOFUR, AMOXYCILLIN AND POTASSIUM CLAVULANATE, AMOXYCILLIN AND CLOXACILLIN, STREPTOPENICILLIN FOR SUSPENSION, AMOXYCILLIN AND CLOXACILLIN, CEFTRIAXONE TAZOBACTUM ETC.
High-Quality Manufacturing
Every injection is produced in Bihar using pharmaceutical-grade ingredients and complies with Indian Pharmacopeia standards, ensuring top-notch quality and safety for clinical use. Our modern facilities support bulk and contract manufacturing to meet demands efficiently.
Customizable Solutions
We offer injectable formulations in a variety of strengths and pack sizes. The choice of container material, vial/ampoule sizes, and brand labeling can all be tailored according to client or market demands, enabling flexibility for different medical or institutional needs.
Safe and Reliable Administration
Our injectables are intended for intravenous or intramuscular use and must be administered by qualified medical personnel only. Comprehensive labeling and instructions help ensure correct and safe usage in hospitals, clinics, and at institutional levels.
FAQs of INJECTION MANUFACTURER IN BIHAR:
Q: How is the injectable solution manufactured and what standards does it adhere to?
A: Our injectable solutions are produced in Bihar, India, using pharmaceutical-grade ingredients under sterile conditions. Each batch is manufactured in compliance with Indian Pharmacopeia standards to guarantee safety and quality.Q: What is the recommended usage of these injectable solutions?
A: These products are intended for intravenous (IV) or intramuscular (IM) administration exclusively by qualified healthcare professionals. Dosage and administration should always follow the prescribing physicians instructions.Q: When should these injectables be used?
A: Injectables are to be used as advised by a medical doctor, typically in hospitals, clinics, or institutional settings. The specific indicationsuch as pain management or infection controldepends on the selected formulation and diagnosis.Q: Where are these injection products available and who can purchase them?
A: Our injectables are manufactured in Bihar, India, and are available for order by healthcare providers, dealers, exporters, wholesalers, and other certified distributors seeking medical solutions for clinical use.Q: What is the process for ordering and customizing these injections?
A: Clients can specify their required formulation, dosage, packaging type, and branding. We accommodate bulk and contract manufacturing, tailoring each batch to the clients detailed specifications, including container material and labeling.Q: What are the key benefits of sourcing injectables from this manufacturer?
A: Clients receive high-quality, compliant products with customizable options, reliable bulk supply, and assurance of sterile, pharmaceutical-grade injectables suitable for a wide range of medical conditions as prescribed by healthcare professionals.Q: How should the injectables be stored and what is their shelf life?
A: All injectable solutions should be stored in a cool, dry place to maintain efficacy. Each vial or ampoule maintains quality for 24 months from the manufacturing date, provided storage instructions are followed.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+













 Send Inquiry
Send Inquiry Send SMS
Send SMS