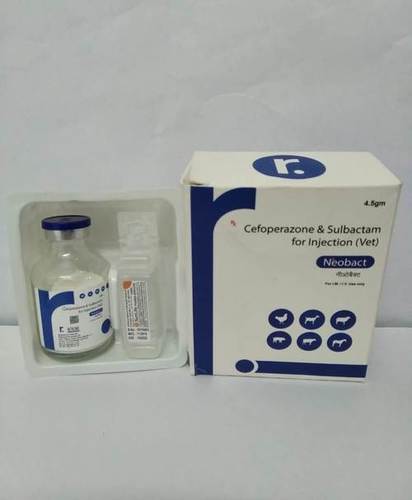
CEFOPERAZONE SULBACTUM INJECTION VETERINARY
334 INR/Bottle
Product Details:
- Shelf Life 24 months from date of manufacture
- Packaging Type Sterile glass vial with separate sterile water for injection ampoule
- Indication Bacterial infections including respiratory tract, urinary tract, reproductive tract, skin & soft tissue, bones & joints, and septicemia
- Medicine Type Antibiotic Injection
- Physical Form Liquid
- Ingredients Cefoperazone Sodium and Sulbactam Sodium
- Treatments & Functions Treatment of infections caused by cefoperazone and sulbactam sensitive organisms
- Click to View more
X
CEFOPERAZONE SULBACTUM INJECTION VETERINARY Price And Quantity
- 1000 Bottle
- 334 INR/Bottle
- White to off-white powder
- To be administered under the supervision of a registered veterinarian
- Milk: 3 days, Meat: 5 days (as per recommendations)
- Manufactured as per GMP norms
- Not to be used in known hypersensitivity to cephalosporin group
- Third Generation Cephalosporin with Beta-lactamase Inhibitor
- Sterile Water for Injection provided
- Single use vial; discard unused portion
- Various veterinary pharmaceutical manufacturers
- Sterile Powder for Injection
- Add sterile water for injection as per label instructions before use
- Intramuscular (IM) or Intravenous (IV)
CEFOPERAZONE SULBACTUM INJECTION VETERINARY Product Specifications
- 24 months from date of manufacture
- Veterinary use only (Cattle, Buffalo, Sheep, Goat, Horses, etc.)
- Cefoperazone 2g + Sulbactam 1g per vial
- Bacterial infections including respiratory tract, urinary tract, reproductive tract, skin & soft tissue, bones & joints, and septicemia
- Cefoperazone Sodium and Sulbactam Sodium
- Liquid
- 3g Vial
- Sterile glass vial with separate sterile water for injection ampoule
- Treatment of infections caused by cefoperazone and sulbactam sensitive organisms
- Store in a cool, dry, and dark place below 25C. Protect from light.
- Antibiotic Injection
- White to off-white powder
- To be administered under the supervision of a registered veterinarian
- Milk: 3 days, Meat: 5 days (as per recommendations)
- Manufactured as per GMP norms
- Not to be used in known hypersensitivity to cephalosporin group
- Third Generation Cephalosporin with Beta-lactamase Inhibitor
- Sterile Water for Injection provided
- Single use vial; discard unused portion
- Various veterinary pharmaceutical manufacturers
- Sterile Powder for Injection
- Add sterile water for injection as per label instructions before use
- Intramuscular (IM) or Intravenous (IV)
Product Description
INJ. CEFOPERAZONE 3G, SULBACTUM SODIUM USP 1.5G( NEOBACT)
CEFOPERAZONE SULBACTUM INJECTION WITH THE BRAND NAME NEOBACT 4.5GM. WE PROVIDE VETERINARY PCD FRANCHISE , VETERINARY THIRD PARTY MANUFACTURING , VETERINARY INJECTION MANUFACTURER, VETERINARY BOLUS MANUFACTURER, VETRINARY ANIMAL FEED SUPPLEMENT, OXYTETRACYCLINE INJECTION, TOP TEN VETERINARY COMPANY, VETRINARY PCD PHARMA FRANCHISE, ETC.
Broad-Spectrum Efficacy for Veterinary Infections
Combining Cefoperazone, a third-generation cephalosporin, with Sulbactam, a beta-lactamase inhibitor, this injection provides robust coverage against a wide range of bacterial pathogens. It is particularly valuable in treating infections unresponsive to regular antibiotics in various livestock species.
Safe, Sterile, and Easy to Use
The formulation is a sterile powder that must be reconstituted with the supplied sterile water. Designed for single use only, it ensures safety, efficacy, and ease of administration for veterinarians. Clear storage instructions and compliance with GMP offer added reliability.
FAQs of CEFOPERAZONE SULBACTUM INJECTION VETERINARY:
Q: How should Cefoperazone Sulbactam Injection be administered to animals?
A: This injection must be administered by a registered veterinarian using either the intramuscular (IM) or intravenous (IV) route, as directed in the dosage guidelines. Reconstitute the sterile powder with the provided sterile water before administration.Q: What types of infections can this injection treat in animals?
A: The injection is effective against various bacterial infections, including those of the respiratory tract, urinary tract, reproductive organs, skin and soft tissue, bones, joints, and septicemia, caused by susceptible organisms.Q: When should the withdrawal period be observed for animals receiving this antibiotic?
A: Milk from treated animals should not be used for 3 days, and meat should not be consumed for 5 days after the last injection, as per the recommended withdrawal periods.Q: Where should the unused and reconstituted portions of the injection be stored or disposed of?
A: Each vial is intended for single use. Any unused reconstituted solution must be discarded and not stored or reused. Store unopened vials in a cool, dry, and dark place below 25C, away from direct light.Q: What is the process for preparing the injection before use?
A: Add the exact amount of sterile water for injection (as indicated on the label) to the vial. Gently mix until the powder is fully dissolved. Use immediately after reconstitution to ensure efficacy.Q: Are there any restrictions or contraindications for using this product?
A: The product should not be used in animals known to be hypersensitive to cephalosporin antibiotics. Administration must always be supervised by a registered veterinarian.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email







 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free